Enhanced Supercapacitor Materials: A Breakthrough in Energy Storage Technology

Image Source: FreeImages
The field of energy storage has witnessed significant advancements with the emergence of enhanced supercapacitor materials. These materials, derived from pyrolyzed algae and graphene composites, offer a unique combination of high power density, fast charge-discharge rates, and long cycle life. They have the potential to revolutionize various industries, including portable electronics, electric vehicles, and renewable energy systems.
The Promise of Supercapacitors
Supercapacitors, also known as electrochemical capacitors or ultracapacitors, have garnered attention as promising energy storage devices due to their exceptional performance characteristics. Compared to traditional batteries, supercapacitors offer higher power density, faster charge-discharge rates, and longer cycle life. This makes them ideal for applications that require quick bursts of energy and frequent charging and discharging cycles.
The performance of supercapacitors primarily relies on the properties of the electrode materials. Carbon-based materials have been widely explored as electrode materials due to their high surface area, good electrical conductivity, and excellent electrochemical stability. Traditional carbon sources, such as activated carbon, have been extensively utilized. However, there is growing interest in developing sustainable and cost-effective electrode materials derived from natural compounds.
Pyrolysis: A Promising Method for Synthesizing Carbon-based Materials
Pyrolysis, a thermal decomposition process in the absence of oxygen, has emerged as a promising method for synthesizing carbon-based materials from natural precursors. This method involves subjecting the precursors to high temperatures, resulting in the decomposition of organic compounds and the formation of carbon-rich materials. Pyrolyzed materials exhibit unique structural and electrochemical properties, making them attractive options for supercapacitor applications.
One of the key advantages of using natural compounds as precursors is their abundant availability, low cost, and environmental friendliness. In recent studies, researchers have explored the pyrolysis of diverse natural sources, such as rice husks, chitosan, and lignin-based materials, to synthesize electrode materials for supercapacitors. These studies have demonstrated the impressive electrochemical performance achievable with bio-waste materials, highlighting the potential of sustainable and environmentally friendly electrode materials derived from readily available natural sources.
Investigating Pyrolyzed Algae and Graphene Composites
In this study, we conducted the synthesis and characterization of supercapacitor materials derived from pyrolyzed natural compounds. Specifically, we investigated four compounds: lysine with methylcellulose, lysine with methylcellulose-graphene composite, algae, and algae-graphene composite. Lysine, an essential amino acid, and methylcellulose, a biodegradable polymer, have shown potential in various applications due to their unique physicochemical properties. Algae, on the other hand, are abundant and renewable biomass resources that have gained attention for their potential use in energy storage systems. The incorporation of graphene, a two-dimensional carbon material with exceptional electrical conductivity and mechanical strength, offers the opportunity to enhance the performance of the resulting supercapacitor materials.
To gain a comprehensive understanding of the synthesized materials, we employed various solid-state physics techniques for characterization. Scanning electron microscopy (SEM) analysis provided crucial insights into the surface morphologies of the composite materials. The SEM images revealed diverse surface morphologies, influenced by the reactants used during the synthesis process. The presence of graphene in the composite materials resulted in distinct structural changes, such as the formation of irregularly shaped carbon structures with a porous surface. These porous structures are significant for supercapacitor applications as they provide ample active sites for charge storage and efficient ion diffusion. Additionally, the presence of graphene enhances the electrical conductivity of the materials, facilitating rapid electron transport during charge-discharge cycles.
Raman spectroscopy, a leading method for characterizing carbon materials, was utilized to analyze the molecular structure and bonding configurations of the synthesized materials. The Raman spectra revealed the presence of various carbon-related vibrational modes, such as the disorder-induced band (D-band) and the band associated with graphene in graphite (G-band). The intensities of these bands were analyzed to determine the number of defects present in the carbon structure and the number of graphene layers. The results indicated the presence of few graphene layers, further confirming the successful incorporation of graphene into the composite materials.
The Brunauer-Emmett-Teller (BET) method was employed to determine the specific surface area (SBET) of the materials. The materials derived from methylcellulose and algae exhibited high specific surface areas, indicating their potential for efficient charge storage. Nitrogen adsorption isotherms and pore size distribution analysis further confirmed the presence of microporous structures in the carbon materials. The micropores, along with the large surface area, contribute to the materials’ high supercapacitor performance by facilitating rapid ion and electron transport.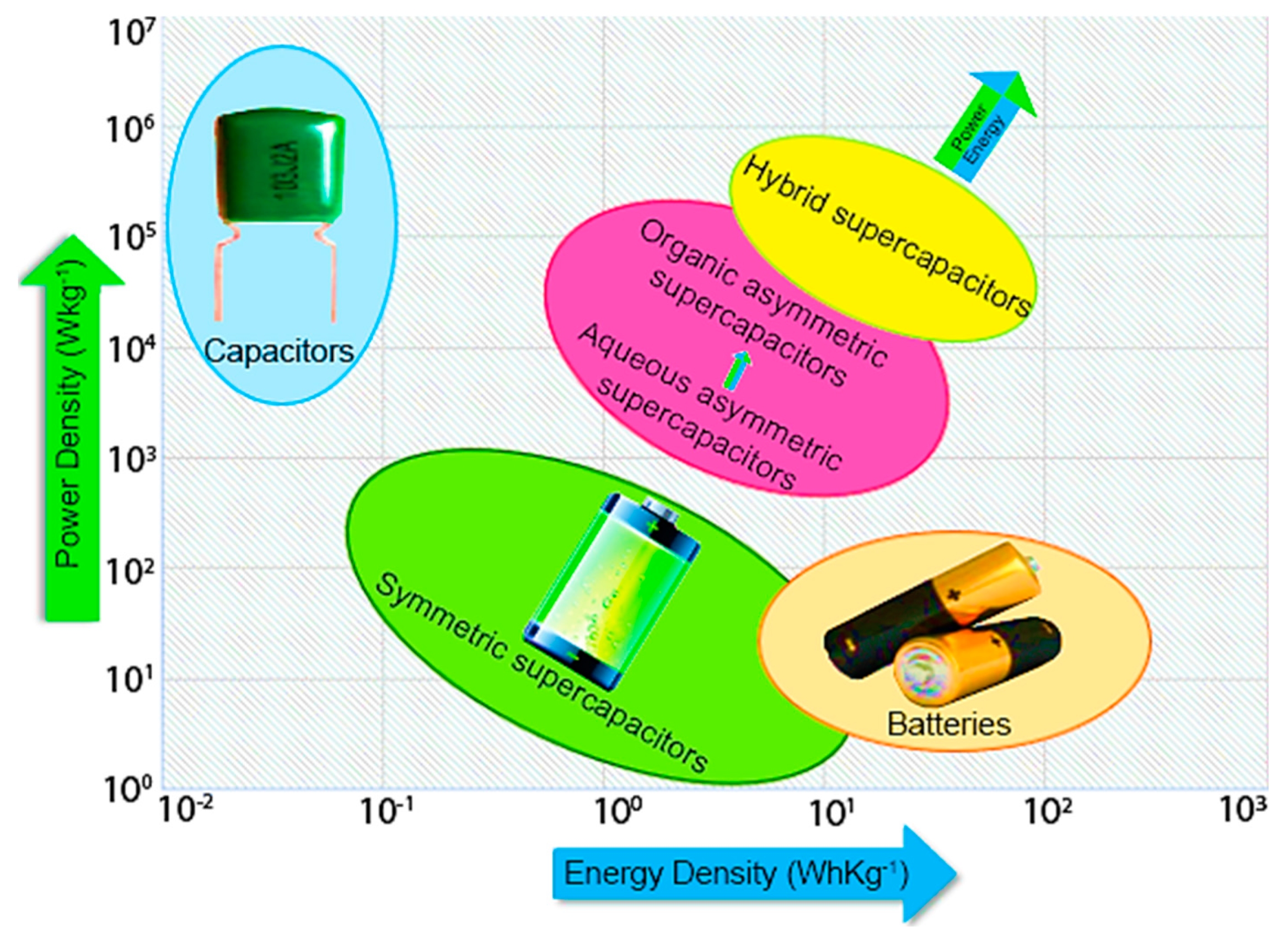
To gain insights into the elemental composition of the materials, elemental combustion analysis was conducted. The results revealed the relative amounts of carbon, nitrogen, and hydrogen present in the carbon-based materials, providing quantitative information about their composition. X-ray photoelectron spectroscopy (XPS) analysis was performed to determine the chemical states of the atoms on the surface of the materials. The high-resolution spectra allowed for the identification of various carbon-containing functional groups and their chemical bonds. These surface analysis techniques provided a comprehensive understanding of the structural and morphological properties of the synthesized materials, facilitating the design and optimization of advanced supercapacitor materials.
Electrochemical Performance of Pyrolyzed Algae and Graphene Composites
To evaluate the electrochemical properties of the synthesized materials, various electrochemical measurements were conducted. In a three-electrode configuration, cyclic voltammetry (CV) measurements were performed to assess the charge storage behavior of the electrode materials. The CV curves exhibited an approximately rectangular shape, indicating the coexistence of electric double-layer capacitance (EDLC) and a reversible Faraday effect. The rectangular shape of the CV curves suggests efficient charge storage within the electric double layer, indicating good capacitive behavior of the electrodes. The reversible Faraday effect observed in the CV curves indicates the occurrence of redox reactions associated with the surface functional groups or heteroatoms present in the carbon materials. These redox reactions contribute to the overall charge storage capacity and enhance the electrochemical performance of the electrodes.
One intriguing finding is the significant enhancement in electrochemical capacitance observed for materials incorporating graphene, despite having a smaller specific surface area than their counterparts without graphene. This unexpected observation challenges the notion that surface area is the sole driver of enhanced performance in supercapacitors. Other factors, such as conductivity and structural characteristics introduced by graphene, may contribute to the increased electrochemical capacitance. The exceptional electrical conductivity of graphene facilitates efficient charge transfer and electron transport within the electrode, enabling a higher capacitive response. Additionally, the unique structural properties of graphene, such as its two-dimensional layered structure and large aspect ratio, create favorable conditions for ion diffusion and accessibility to active sites, thereby enhancing the charge storage capability. The incorporation of graphene has opened up new avenues for improving the electrochemical performance of supercapacitor materials, demonstrating that factors beyond surface area significantly influence the capacitance of electrode materials.
The electrochemical impedance spectra (EIS) analysis further confirmed the positive impact of graphene on charge transfer kinetics within the electrode-electrolyte interface. The absence of a semicircle in the high-frequency region and the decreased slope in the low-frequency region indicated a relatively small charge transfer resistance and a transition from purely capacitive to pseudocapacitive behavior, respectively. The presence of graphene contributed to a reduction in resistance, enhancing the overall performance of the supercapacitor materials.
To assess the long-term stability of the synthesized materials, electrochemical measurements were conducted over 1,000 charge-discharge cycles. The results demonstrated the excellent cyclic stability and long-term performance retention of the materials with graphene additives. Although a gradual decrease in capacitance was observed after a certain number of cycles, the capacitance level remained exceptional and higher than the initial value even after 1,000 cycles. This indicates the enduring stability and sustained performance of the materials over an extended period.
In a two-electrode configuration, galvanostatic charge-discharge (GCD) measurements were performed to evaluate the charge storage and discharge behavior of the electrode materials. The charge-discharge curves exhibited a symmetrical quasi-triangular shape even at high current densities, indicating the high specific capacitance and efficient energy storage capability of the materials. The relationship between capacitance and charge/discharge currents revealed a similar trend of capacitance decrease with increasing current. However, the materials exhibited a remarkable capacitance retention throughout the cycling test, indicating their stability and durability in a symmetric supercapacitor configuration.
To evaluate the long-term stability of the materials, a floating test was conducted. The test involved subjecting the symmetric supercapacitor to a 100-hour floating period at a specific voltage and current density. The results demonstrated the stability of the cell voltage within the specified range, with a capacitance loss of approximately 22% after 100 hours of floating. This observation further emphasized the enduring stability of the materials, even under prolonged operational conditions.
Conclusion
Enhanced supercapacitor materials derived from pyrolyzed algae and graphene composites offer a promising solution for energy storage applications. These materials exhibit high power density, fast charge-discharge rates, and long cycle life, making them suitable for various industries. The synthesis and characterization of these materials have been extensively investigated, revealing their unique structural and electrochemical properties.
The incorporation of graphene into the pyrolyzed natural compounds significantly enhances the electrochemical performance of the resulting supercapacitor materials. Despite having a smaller specific surface area, the addition of graphene leads to improved capacitive behavior, higher specific capacitance, and excellent cyclic stability. The exceptional electrical conductivity of graphene facilitates efficient charge transfer and electron transport, while the unique structural properties create favorable conditions for ion diffusion and accessibility to active sites.
These findings highlight the significant role of graphene in enhancing the electrochemical properties of supercapacitor materials. The development of enhanced supercapacitor materials derived from pyrolyzed algae and graphene composites opens up new avenues for energy storage technology. With further research and optimization, these materials have the potential to revolutionize various industries and contribute to the advancement of sustainable and environmentally friendly energy storage solutions.